Understanding the love-hate relationship of halide perovskites with the sun
New research by scientists at TU/e and universities in China and the US sheds light on the causes of perovskite solar cell degradation.
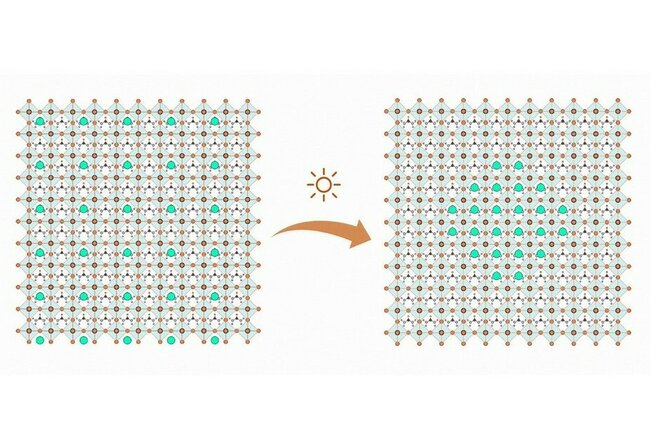
Solar cells made of perovskite are at the center of much recent solar research. The material is cheap, easy to produce and almost as efficient as silicon, the material traditionally used in solar cells. However, perovskite cells have a love-hate-relationship with the sun. The light that they need to generate electricity, also impairs the quality of the cells, severely limiting their efficiency and stability over time. Research by scientists at TU/e and universities in China and the US now sheds new light on the causes of this degradation and paves the way for designing new perovskite compositions for the ultimate stable solar cells.
Perovskite is an attractive alternative to silicon, because it’s abundant and easy to produce. What’s more, over the past decade, the performance of perovskite solar cells has improved dramatically, with efficiency rates reaching more than 25 percent, which is close to the state-of-art for silicon solar cells.
The new research focuses on perovskite solar cells made from formamidinium-cesium lead iodide, a halide compound that has become increasingly popular as it combines high efficiency and reasonable heat resistance with low manufacturing costs.
Love-hate
However, solar panels made of this particular compound have a rather ambivalent relationship with sunlight, a problem that is well-known in the field, but barely understood. While the light of the sun feeds it with the much-wanted energy to convert into electricity, it also impairs the stability of the cells. Over time this affects their performance.
To understand why this is the case, the researchers at TU/e, Peking University and University of California San Diego did both practical experiments – monitoring the photovoltaic performance of the panels over 600 hours of exposure and characterizing the degraded perovskites – and theoretical analysis .
From this they conclude that sunlight generates charged particles in the perovskite, which tend to flow to places in the solar panel where the band gap (the minimum amount of energy needed for generating the free electrons) is lowest, in this case the formamidinium perovskite. The resulting energy differences make the mixed compounds that worked together so well to make the cell efficient, fall apart into separate clusters. It appears that especially the cesium-heavy clusters (the green dots in the image) are photoinactive and current-blocking, limiting the performance of the device.

Solutions
According to Shuxia Tao, who together with PhD candidate Zehua Chen and her colleague Geert Brocks was responsible for the TU/e part of the research, the new findings are one step further to finding the way to possible solutions.
“By combining macroscopic tests, microscopic materials characterization and atomistic modelling, we were able to thoroughly understand the instability of halide perovskites that are intrinsic to device operation. This opens the possibility for designing new perovskite compositions for the ultimate stable solar cells.”
Possible strategies include using additives to enhance the chemical interaction inside the materials in the panels, tuning the band gaps by using other elements like bromide and rubidium instead of iodide and cesium, or modifying the energy levels to extract photo-carriers more efficiently.
Tao stresses that more research is needed to see what solution works best. In addition, separation of halide compounds is not the only cause for perovskite degradation. These additional causes require separate analysis.
The results are published in the scientific magazine Joule.
N. Li, Y. Luo, Z. Chen, G. Brocks, S. Tao, D. P. Fenning, H. Zhou, et al. Microscopic Degradation in Formamidinium-Cesium Lead Iodide Perovskite Solar Cells under Operational Stressors, DOI: 0.1016/j.joule.2020.06.005.