Producing sustainable fertilizer compounds
Dimitra Anastasiadou defended her thesis at the Department of Chemical Engineering & Chemistry on September 14th.

Nitrogen pollution is a hot topic in the Netherlands at the moment. Some natural areas suffer from high nitrogen levels, while the agricultural industry is still very reliant on fertilizers. In her PhD research, Dimitra Anastasiadou focused on synthesizing green fertilizers by utilizing nitrogen waste compounds.
Ensuring a fertile growing environment for crops becomes crucial as agricultural demands continue to rise. Crops absorb vital nutrients from the soil during their growth, and these nutrients must be replenished to maintain optimal productivity.
Otherwise, crop production may stagnate or even dwindle. Fertilizers play a pivotal role in providing essential nutrients such as phosphorus, potassium, and nitrogen, which are essential for robust plant growth, disease resistance, and ultimately, sustainable crop production.
Nutrients
Among these nutrients, fixed nitrogen is the primary macronutrient that often limits agricultural yield. As a result, synthetic nitrogen-based fertilizers like ammonia, nitrate, and urea have become indispensable in meeting global food demand.
The inception of ammonia production by Haber and Bosch in the early 20th century revolutionized agriculture and enabled exponential population growth. Today, annual ammonia production through the Haber-Bosch process stands at approximately 176 million tons, with projections indicating a significant expansion to 1.2 billion metric tons by 2050, owing to its diverse applications in various industries.
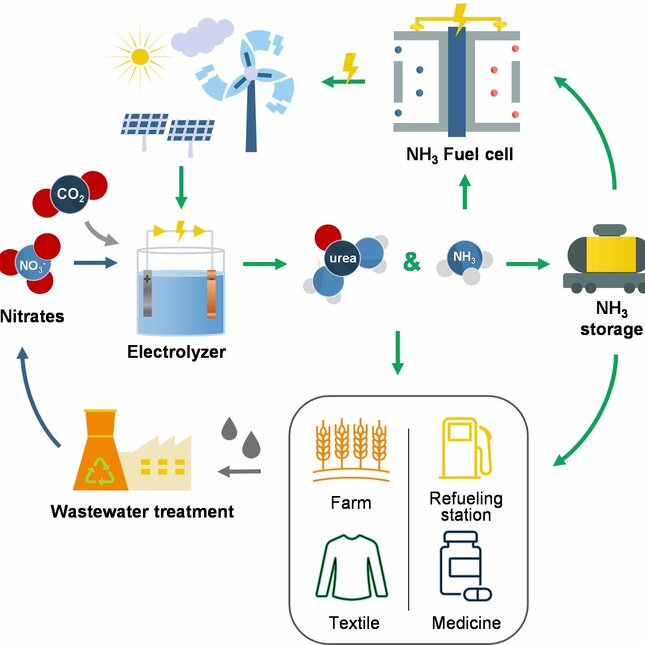
Urea, a crucial downstream product of ammonia, finds widespread application in agriculture, serving as a vital raw material for pesticides and medicines. In fact, urea-based fertilizers account for an impressive 70% of global nitrogen-containing fertilizers.
Environmental concerns
While synthetic fertilizers have played a crucial role in food production, their production process relies heavily on fossil fuels, contributing to environmental concerns. In response, the energy transition towards sustainable and environmentally friendly practices gains importance.
Electrochemical processes, which harness renewable electricity to store energy and convert it into chemical bonds, offer promising solutions. These processes not only enable the production of chemical building blocks and fuels from renewable resources but also promote cleaner energy generation and sustainable chemical production. Notably, the falling prices of renewable electricity facilitate the development of cost-effective clean power systems.
The shift from fossil fuel-based synthesis of nitrogen-based fertilizers to more sustainable alternatives is critical for the energy transition. In light of this, our research focuses on harnessing electrochemical conversion to transform environmentally harmful molecules, like nitrates and carbon dioxide, into valuable compounds such as ammonia and urea.
To facilitate this process, Anastasiadou explores the use of appropriate materials (catalysts) that reduce energy requirements and ensure stable performance throughout the conversion process.
Key objectives include synthesizing and evaluating various materials to determine their efficacy in selectively and efficiently producing targeted products. By delving into the role of these materials, the research seeks to optimize the conversion of starting compounds into the desired end products.
Ultimately, her PhD research contributes to the development of sustainable, energy-efficient, and environmentally friendly methods for nitrogen-based fertilizer production, thus fostering a greener and more resilient agricultural future.
Conclusions
The synthesis of nitrogen-based fertilizers such as ammonia and urea were synthesized with electrochemical methods by utilizing waste compounds like carbon dioxide and nitrates. The question of developing an efficient catalyst for urea electrosynthesis was thoroughly investigated.
Non-precious metals like copper and zinc were used as catalysts. The bimetallic system of copper and zinc oxides was for the first time methodically studied by varying the metal ratios for the electrosynthesis of urea.
Anastasiadou’s research presented an FE to urea of 41%, placing the tested catalyst amongst the most efficient reported for urea electrosynthesis from carbon dioxide and nitrate. Despite this promising result, many other aspects need to be addressed before such a catalyst can be implemented in practice.
Furthermore, her thesis largely focuses on understanding the catalytically active sites for urea electrosynthesis. Understanding the state and interaction of the bimetallic catalysts during the reduction and linking them to the observed electrocatalytic properties is valuable for a rational design of efficient electrocatalysts for urea synthesis.
A selectivity and activity dependence on the relative composition of the copper and zinc oxide catalysts was found and attributed to a synergetic electronic effect. Regardless of these recent developments in the field, further research is needed to clarify the underlying reaction pathways and improve catalyst engineering.
Title of PhD thesis: “Cu-based electrodes for ammonia and urea electrosynthesis”
Supervisors: Emiel Hensen, Marta Costa Figueiredo