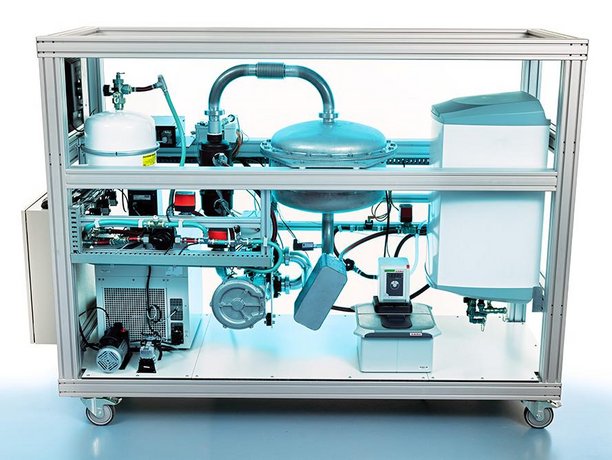
At the moment, energy companies are primarily focused on developing heat networks as a means to disconnect districts from the natural gas grid. ‘The problem with heat networks is that they are far from flexible, and suffer from heat losses that reduce efficiency,’ state Adan and Huinink. Over the past decade, together with TNO, TU/e’s research group Transport in Permeable Media (TPM) developed a flexible and loss-free alternative based on salt hydrates. The basic idea is simple: you take a chunk of hydrated salt and add some heat to it. The heat is used to evaporate the water, leaving you with dry salt. As soon as you expose the salt to water again, the heat will be rereleased. As long as the salt is kept somewhere dry, the heat is stored in a loss-free way, if necessary for weeks or months on end.
‘Our solution is not only loss-free, but it can also be used to harvest the heat where it is produced and bring it to the place where it is needed,’ says Huinink. ‘There is no need for the two locations to be physically connected. And it is this type of flexibility we desperately need in this phase of the energy transition.’ After all, it makes little sense to build an extensive and expensive heat network connected to a coal-fired power station, which is bound to be phased out any time in the near future.
Unlocking unused potential
Furthermore, the salt-based system can be operated under relatively low temperature conditions, unlocking heat sources that are now often discarded, adds Adan. ‘Take the residual heat produced by a datacenter. This is often regarded to be too low temperature to be of use. But with our technology, we can use this energy to warm up tap water to the required level of some sixty degrees Celsius.’ In the Netherlands alone, low temperature (i.e. below 150 0C) residual heat from industry accounts for some 150 petajoules, enough to heat six million homes, Adan lectures. And since the salt has a very high energy density, you can store that heat in relatively small volumes.
For TU/e and TNO, the joint research on so-called thermochemical materials started some ten years ago, Adan recollects. ‘Around 2008, we recognized heat storage and transport to be a potential barrier in the energy transition. In 2010 we hired our first PhD student to start exploring the possibilities of using thermochemical materials to this end.’ Huinink: ‘The concept of using salt and water is in fact quite old. People have been working on this in the seventies, at the time of the oil crisis. But all of their attempts to turn this idea into a useable technology failed. In hindsight, the problem was that none of them ever covered the entire chain from fundamental research to the end user.’
Timeline
- 2008 - TPM group develops first ideas on research into thermochemical materials
- 2010 - First PhD student explores challenges and possible solutions for heat storage
- 2015 - CREATE project receives five million euro grant from EU Horizon 2020 program
- 2018 - Start of TKI Urban Energy funded Cap4Heat and Dope4Heat projects and NWO funded Mat4Heat project to improve the performance and stability of the salt hydrate
- 2019 - Patent granted on closed loop reactor design
- 2019 - HEAT-INSYDE project receives seven million euro grant from the EU Horizon program
- 2020 - Start-up Cellcius founded
- 2022 - Field experiments with heat batteries in residential areas in the Netherlands, France and Poland
- 2022 - Field experiments with heat transport together with Chemelot
Heat batteries for homes
The Eindhoven researchers decided to take a different turn, and to always keep the final application in mind during the development process. They aimed to develop a heat battery for households, which stores excess renewable energy, for example generated by solar panels on sunny days, and use it to heat the tap water later on. Huinink: ‘After having defined the most important questions and most suitable material candidates, in 2015 we started a project to delve into the materials science and find out how the properties of the different salt hydrates we could use would map out on the envisioned operating conditions.’ They ended up with potassium carbonate (K2CO3) as the most suitable base material to do the job. This salt has such a high energy density that a refrigerator-size battery should be sufficient to supply a medium household with warm tap water for about two weeks. Furthermore, the salt is neither too expensive nor toxic, and it is made from abundantly available resources.
The researchers built a first demonstrator to convince external parties of the merits of their idea. That concept will be now tested in Eindhoven, the South of France, and Poland to assess how different configurations – combining the heat battery with heat pumps, solar panels or solar collectors – work in different climates and how users reflect on the system. On top of that, the researchers aim to also experiment with heat transport together with Chemelot later this year. In the meantime, to further accelerate the time to market of this innovation, start-up company Cellcius will translate the findings from the academic research to products and services. This does not mean the fundamental science part of the work is over, Huinink stresses. ‘On the material side, we are working on further improvements of the salt’s performance. To that end we are delving into the fundamentals of the hydration and dehydration process, and into the flow dynamics of the water vapor we use to hydrate the salt. At the same time we are still working on the material composition to further improve stability and increase its lifetime.’
Move fast
‘Looking back, we managed to solve two major problems in a remarkably short period of time,’ Adan concludes. ‘First, we developed a potassium carbonate based salt composite that does not disintegrate nor coagulate during hydration and dehydration, enabling it to maintain its stability over multiple charging and decharging cycles. And second, we came up with a patented system design that makes optimal use of the properties of the selected material.’ The fact that the group came this far in only ten years is, besides to the occasional serendipity, also largely due to their systematic approach, both men agree. ‘We literally took a car and drove around Europe to visit groups working on similar concepts to assess upfront what would and would not work. And on the side, we have been setting the national and European agendas for heat-related research. As a result we were able to secure funding from large scale energy-related programs we partly shaped ourselves. And finally, the fact that our group is a part of a thriving ecosystem of collaborating academia, knowledge institutes and industry has surely enabled us to move fast.’