A FAST TRACK TO CLINICAL INNOVATION
The established healthcare systems are under pressure. Ageing society, increasing costs and growing workload for a reduced number of healthcare professionals pose extensive social and economic challenges. An integral approach to healthcare innovation is required, involving many disciplines working together in an early stage and providing structural support for these challenges. Such collaboration requires sharing data, implementation strategies, and teamwork across the entire value-chain with a joint focus towards “a fast track to clinical innovation”.
Vision & Mission
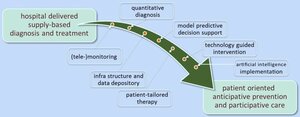
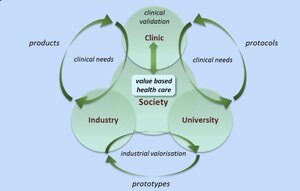
Fast implementation of dedicated high-tech health care innovations will be indispensable to maintain a cost-effective healthcare system that is configured around what individual patients really need for a better health care outcome. The e/MTIC mission is to drive value-based healthcare by growing an ecosystem that creates a fast track in research, development and implementation of sustainable innovations in clinical practice by strengthening the institutionalized collaboration between regional partners focusing on research and innovation in pre-defined clinical domains.
Organisation
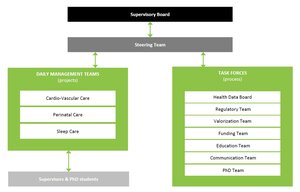
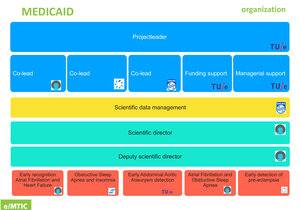
e/MTIC has been officially launched in June 2018. At the end of 2019, e/MTIC’s organisation was formalized with three Daily Management Teams (DMTs) that established the roadmaps for the Cardiovascular, Perinatology and Sleep application domains. Additionally, six Process teams (Taskforces) were appointed to support the e/MTIC activities. Both DMTs and Taskforces have put in place organisation charters, working-processes and their own KPI’s.
The e/MTIC community has expanded significantly and today counts over 150 researchers, staff and other contributors. The innovation ecosystem attracts many research talents and delivers tangible results for clinical innovation with the support of Dutch funding. e/MTIC positions itself as the partner to bring healthcare innovation ideas to clinical testing. As such, the anticipated impact of the ecosystem will be of great importance for different partners.
Governance
Because of the magnitude and breadth of the above ambitions, a broad and balanced multi-level governance structure is being established. A backbone of (around 35) senior bilateral cross-appointments, of key clinical specialists and industrial experts as part-time full professor at TU/e ensures that all partners are continuously well aligned. Additionally, key TU/e and clinical experts have taken up a formal advisory role in industry. Clinical PhD students are teaming up with engineering PhD students, to reinforce each other and each PhD student is employed part-time at the partner institution(s) working under daily supervision.
The three DMT's ensures that the program runs smoothly, stays on track, remains aligned with strategic directions, and produces scientific, clinical, and industrial impact.The overall operational and strategic management is handled by a Steering Group composed of senior managers and researchers from all five partners. The full e/MTIC collaboration is overseen by a Supervisory Board at an executive management level which meets twice a year to review progress, resolve strategic issues, and provide strategic guidance.
Dedicated joint Task Forces proactively support the e/MTIC collaboration in areas of common interest across DMT’s such as clinical data acquisition and sharing, education, clinical regulatory matters and acquisition of external funding.
Read more about the e/MTIC Task Forces
e/MTIC annual program
Each year a new e/MTIC program is added to the portfolio, based on the roadmaps and based on funding by the partners. This allows the e/MTIC consortium to carry out a coherent program within the e/MTIC ecosystem with a succession of yearly defined projects (each having a duration of 4 -5 years). One of the main goals of e/MTIC is to make sure that in four years from now for each application domain, at least one new product or process is implemented in the clinic and is creating impact for patients, applying Value-Based HealthCare (VBHC).
Conclusion
e/MTIC, with its unique ecosystem, provides all essentials for such outcome-driven research with the development of data infrastructure (health data portal), leverage of artificial intelligence, regulatory and valorisation support, cross-appointments and participation of scientific, clinical and business partners.
Embedded in the innovative Brainport region, e/MTIC contributes to technological breakthroughs that improve the quality of life. At the same time, it fuels the necessary adjustments in educational programs that will produce the new generation of MedTech researchers and professionals.