In the flow of energy research
Originally trained as a chemical engineer, current Chair of the Power & Flow group at TU/e’s department of Mechanical Engineering Niels Deen has ample expertise in process technology. This comes in handy in his current EIRES-related research, where he is working on so-called multiphase flows. In a multiphase flow, materials with different thermodynamics phases, such as liquid, gas or solid, are flowing simultaneously. ‘Currently, I am working both on metal fuels and electrolysers. Though those might seem two entirely different things, in my case they both come down to the same: understanding how small, round objects interact with their surroundings.’ In case of metal fuels, Deen is not focusing on the combustion part, but rather on how to reduce the resulting iron oxide back to iron powder, which can then be reused. For the electrolysis part of his research, he focuses on the dynamics of the hydrogen bubbles that are formed when water is split in an electrolyser.
Bubble basics
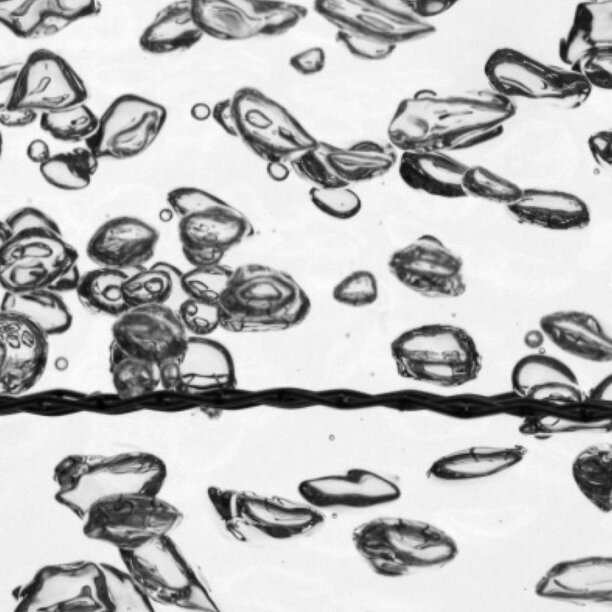
Though his research is application-inspired, Deen’s group aims for a fundamental understanding of the processes that drive energy conversion. He gives an example: ‘We recently published an article that explains flow phenomena on the surface of hydrogen bubbles in an electrolysis set-up. The chemical reaction that leads to the formation of hydrogen gas is exothermic. Previously, people thought that the dissipated heat would cause variations in the surface tension of the bubbles, which in its turn would result in the observed bubble behavior. But that theory did not explain all of the experimental observations. So we delved into that, and found out that insoluble surfactants transport along the bubble interface to form a stagnant cap. This cap causes the observed flows on the bubble surface.’
Besides being of fundamental interest, this knowledge is also relevant for applications, Deen says. ‘At the moment, the efficiency of hydrogen electrolysis isn’t very high. One of the problems is that the hydrogen bubbles stick to the electrodes, preventing new bubbles from being formed. When we understand what causes this sticking behavior, we can start thinking about solutions to remove the hydrogen faster, and thus increase the efficiency of the production process.’
Reducing rust
Also when it comes to his research on metal fuels, there are ample fundamental questions Deen is eager to answer. The idea EIRES researchers are working on is to burn iron powder, leaving iron oxide, or rust, as a residue. Deen looks into the process of transforming the rust back into iron powder, by bringing it into contact with hydrogen gas. ‘The rust consists of powder particles. But how do you make sure the hydrogen can react with all of the iron oxide, and not only with the oxide at the particles’ surface? To answer that question, you need to know how for example diffusion processes within the particles are influencing their ability to react with the hydrogen.’
Another open question is what the optimal process parameters are. ‘In principle goes, the higher the temperature, the faster the reaction. But when you heat the powder too much, the particles will stick together, decreasing their contact area. So we also look into the sticking process itself. How can we best describe that? Those kinds of questions are not only relevant for the metal fuel research, but also for all other applications that make use of powders, like powder coating.’
Besides studying processes on the level of individual particles or bubbles, together with Giulia Finotello, Deen also looks at the bigger picture, for example by studying reactor concepts. ‘What types of reactors can we use to reduce the iron oxide? What is the best way to bring the hydrogen into contact with the powder? How can we keep all particles moving? What is technically feasible, and economically viable?’
For his experiments, Deen makes use of a myriad of different techniques. ‘With high speed camera’s we follow in great detail what is happening. But we also use optical microscopes to study the surface of the hydrogen bubbles or the individual powder particles. And in our Future Fuels lab, we can build prototypes of metal fuel burners and fluidized bed reactors to test our reactor concepts.’
Interplay with students
Though as a scientist, he is primarily driven by curiosity, being able to contribute to the energy transition is an additional motivation for Deen. ‘Working on societal relevant topics also has a great appeal on students, especially at our department of Mechanical Engineering.’ Take student team SOLID that puts ideas about metal fuels to the test where Deen is involved as an adviser. ‘What I like about student teams is that they aim to end up with some concrete result in only one year. It is very stimulating to see what these motivated young people are able to pull off in such a short time. And during their work, they often stumble upon questions that are interesting for me to incorporate in my research.’
Also to be part of EIRES has a great added value for his work, Deen says. ‘I zoom in on a very small piece of the puzzle, for example when it comes to the electrolysers. Within EIRES, I get into contact with people from other departments working on other pieces of that same puzzle. On my own I will not be able to make a real change. But when we join forces throughout the entire university, I am convinced that we can really make an impact.’