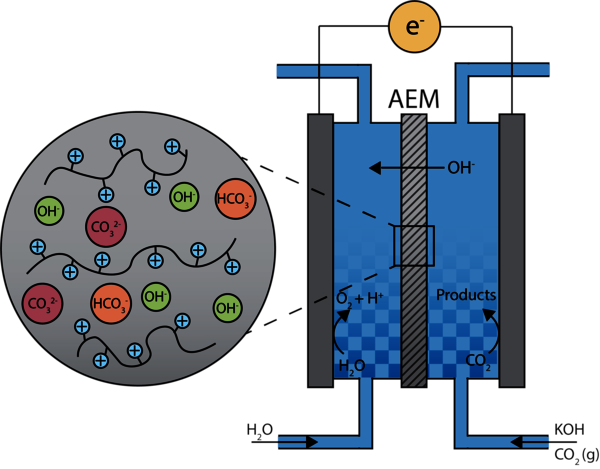
Electrochemical reduction of CO2 towards fuels and chemicals (e.g. ethanol and ethylene) is a promising route to transition to a more sustainable chemical industry and close the carbon cycle. The electrochemical CO2 reduction reaction (CO2RR) is generally performed in reactors consisting of an anode and cathode on opposite sides of an anion exchange membrane (AEM) in an alkaline electrolyte solution (Figure 1). The AEM is a key component for CO2 reduction and consists of a polymer backbone with positively charged groups. It should facilitate the transport of hydroxide ions between the anode and cathode while serving as a barrier between the two compartments.
While extensive research has been performed on optimizing the electrodes for CO2RR, the AEM is less well studied and suffers from poor chemical and mechanical stability at high pH, low hydroxide conductivity and cross-over of negatively charged species. We aim to improve the understanding of membrane influences on the selectivity and efficiency of the CO2RR. This insight will be used to develop a novel AEM with improved chemical stability, hydroxide conductivity and selectivity. Satisfying these requirements is challenging due to the trade-off between the membrane properties. Incorporating additives such as (functionalized) silica could improve the membrane stability without sacrificing the conductivity and selectivity.
Contact details

Name: Woutje ter Weel
Country of origin: The Netherlands
Room: STO 0.48
Email: w.t.weel@tue.nl
TU/e phone: +31402478534