The aging population and increases in chronic diseases put high pressure on the healthcare system, which drives a need for easy to use and cost effective medical technologies. In-vitro diagnostics (IVD) plays a large role in delivering healthcare and within the IVD market decentralized diagnostic testing, i.e. point-of-care testing (POCT), is a growing segment. Applications for which POCT is very relevant are for example the detection of proteomic markers to diagnose cardiac diseases and the detection of nucleic acid markers in case of infectious diseases.
POCT devices should be compact and fully integrated for maximum ease of use. This has sparked developments in the design of integrated lab-on-a-chip biosensing devices. The microfluidic systems have high surface-to-volume ratios, therefore capillary forces and laminar flow strongly impact the transport processes. As a consequence, unconventional techniques need to be applied in order to induce effective and reliable mixing, catching, transportation, and detection of low amounts of biological target material.
A new class of POCT devices is based on magnetic nanoparticles [1, 2]. Important advantages of using magnetic particles are that they have a large surface-to-volume ratio, are conveniently biofunctionalized, and that they can be manipulated by magnetic fields for full control of the integrated biosensing assay.
We investigate the use of magnetic particles and magnetic fields to effectuate mixing, catching as well as detection processes. The rate at which an analyte is captured from a solution is determined by volume transport (translation diffusion), alignment of the analyte’s binding site with the receptors on the surface (rotational diffusion), and biochemical binding. We develop magnetic actuation protocols involving translation and rotation of magnetic capture particles and investigate the ensuing effects on the target capture rate [3]. Moreover, we investigate magnetic particle-particle interactions to control the (dis)aggregation of particles. Chain-like aggregates of particles are used to induce chaotic mixing and counter target depletion near surfaces [4], whereas disaggregation isolates the particles and prepares them for further lab-on-chip processing [5].
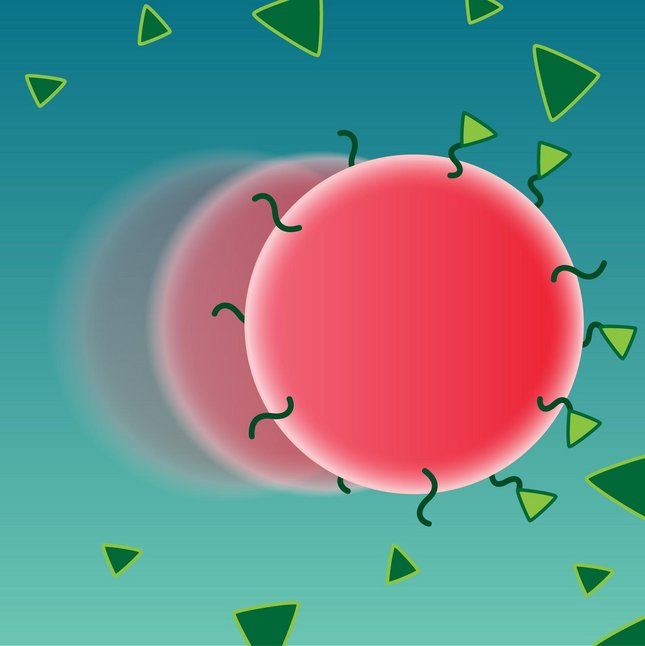
Furthermore, we investigate a novel biosensing technology based on rotating magnetic particles [6]. The presence of analyte molecules induces the clustering of functionalized particles, resulting in particle dimers at low target concentrations and multimers at high concentrations. By applying a rotating magnetic field and recording intensity modulations of the scattered laser light we specifically detect the particle dimers. The modulation depth is an indicator for the fraction of dimers in solution, and thus the analyte concentration. This detection method is fast and sensitive, and has been shown to operate in small volumes of undiluted blood plasma [7].
References
[1] D. M. Bruls, T. H. Evers, J. A. H. Kahlman, P. J. W. van Lankvelt, M. Ovsyanko, E. G. M. Pelssers, J. J. H. B. Schleipen, F. K. de Theije, C. A. Verschuren, T. van der Wijk, J. B. A. van Zon, W. U. Dittmer, A. H. J. Immink, J. H. Nieuwenhuis and M. W. J. Prins, Rapid integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles, Lab on a Chip 9, 3504-3510 (2009).
[2] R.C. den Dulk, K.A. Schmidt, G. Sabatté, S.Liébana, M.W.J. Prins, Magneto-capillary valve for integrated purification and enrichment of nucleic acids and proteins, Lab Chip 13, 106-118 (2013).
[3] A. van Reenen, A.M. de Jong, and M.W.J. Prins, Accelerated particle-based target capture – The roles of volume transport and near-surface alignment, J. Phys. Chem. B 117, 1210-1218 (2013).
[4] Y. Gao and A. van Reenen, M.A. Hulsen, A.M. de Jong, M.W.J. Prins, J.M.J. den Toonder, Chaotic Fluid Mixing by Alternating Microparticle Topologies to Enhance Biochemical Reactions, Microfluid. Nanofluidics 14, 1-10 (2013).
[5] Y. Gao and A. van Reenen, M.A. Hulsen, A.M. de Jong, M.W.J. Prins, J.M.J. den Toonder, Disaggregation of microparticle clusters by induced magnetic dipole-dipole repulsion near a surface, Lab Chip 13, 1394-1401 (2013).
[6] A. Ranzoni, J.J.H.B. Schleipen, L.J. van IJzendoorn and M.W.J. Prins, Frequency-selective rotation of two-particle nanoactuators for rapid and sensitive detection of biomolecules, Nano Lett. 11 2017-2022 (2011).
[7] A. Ranzoni, G. Sabatte, L.J. van Ijzendoorn, and M.W.J. Prins, One-step homogeneous magnetic nanoparticle immunoassay for biomarker detection directly in blood plasma, ACS Nano 6, 3134-3141 (2012).