Description
A novel microfluidic breast cancer model to study the relation between the Notch pathway and mechanotransduction in metastasis
The majority of breast cancer related deaths are not caused by the primary tumor, but by metastases to other tissues. However, due to our limited understanding of this process, preventing or curing metastases remains a major challenge. In this project, the main goal is to further increase our understanding by investigating the poorly understood link between tissue mechanics and cancer metastasis. More specifically, we aim to study the involvement of the Notch signaling pathway in the onset of breast cancer cell invasion.
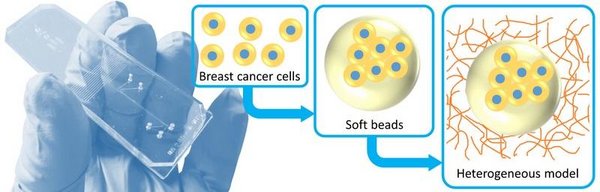
A major challenge is that conventional (2D) cancer models are lacking the relevant physical cues provided by a three-dimensional matrix: "Normal" tumor tissue properties, such as the stiffness and fibrous structure are absent. Additionally, the heterogeneous distribution of these properties throughout a tumor are not incorporated: During the onset of invasion, cancer cells encounter both the soft, laminin-rich basal membrane and the stiffer, more collagenous stromal extracellular matrix (ECM).
In order to overcome these challenges, we aim to develop a novel breast cancer model that incorporates the relevant properties of the three-dimensional microenvironment. For this purpose, we use microfluidic technology, which enables us to manipulate and control fluids at the small scale. Cell encapsulation is used to first generate soft, cell-containing beads that mimic the basement membrane. These beads are then embedded in a more fibrous matrix that mimics the stromal ECM, completing the tissue model.
The physical properties and tissue dimensions can be controlled by varying the process conditions and composition, for which the microfluidic approach is well suited. An additional advantage is that many different conditions can be screened using only small volumes of reagents. However, the most important property of the model is that parameters of the physical environment can be varied individually, enabling systematic investigation of their influence on cancer cell invasion.
The project is a collaboration between prof. dr. Cecilia Sahlgren, part time assistent professor in the STEM group, and prof. dr. ir. Jaap den Toonder of the Microsystems group, in the context of the StressFate project that is funded by the European Commission.
Researchers
Researcher: J.J.F. (Jelle) Sleeboom.
Supervisors: C.M. (Cecilia) Sahlgren, J.M.J. (Jaap) den Toonder.
Contact
-
ir. Jens Sambokand. Jip Bijlsma MACharonlaan1111LW Moerdijk
-
Esther de Ruiterdr.h.c. Naud Weijland LLBVerbeekbaan1775BR Wedde
-
Adam PrinsDaan Schiffervan der Meulensingel1406MZ Den Helder