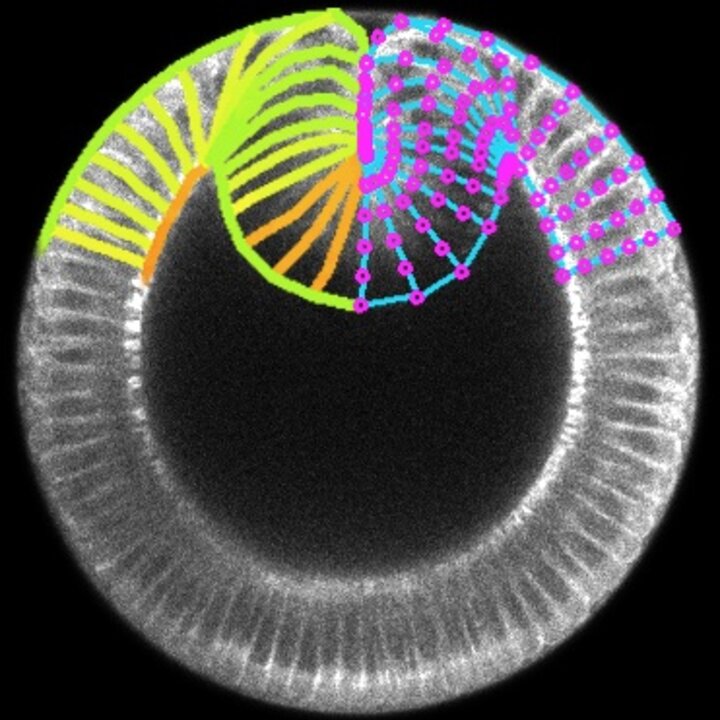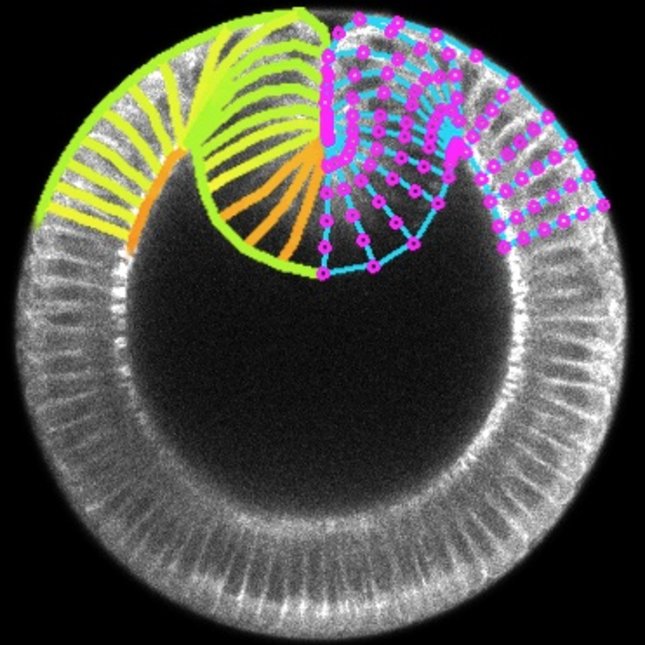
In bioengineering, we aim to regenerate tissues and organs by controlling the behaviour of single cells at the molecular and mechanical levels. However, achieving the formation of specific tissue structures (and functions) requires understanding and controlling various factors such as signalling, shape, force, adhesion, and movement of cells. While we have made significant progress in understanding and controlling these factors individually and combinedly, research suggests that tissue form and function also emerge from the very interaction of multiple cells rather than just the mechanobiological properties of individual cells. Therefore, our main aim consists in identifying the rules of mechanobiological interactions that enable a group of cells to develop into tissue with a predefined shape and function. This knowledge will provide us with basic synthetic design principles for tissue and organ regeneration and help us understand how certain diseases progress as tissues undergo morphological pathological changes. To answer this question, we work on two fronts: developing in vitro techniques to measure how mechanics of cell collectives varies with individual cell biology, which mainly involves 2D traction force microscopy on (visco)elastic (bio)material substrates, and building in silico models to predict how tissue shape and function varies with cell mechanics at both the individual and collective levels, which mainly involves vertex models.