Kidney disease is a major health issue worldwide [1], in the Netherlands alone, more than one in ten individuals has chronic kidney disease (Dutch Kidney Foundation 2016b) and this number is expected to increase in both the short and long term [2]. The risk of kidney failure is still underestimated [3]; moreover, there are no representative in-vitro models that are able to study kidney functionality and screening for drugs. Hence, there is a great demand for such models.
Organ-on-chip is an ideal tool to study human disease [4] and has great potential in biomedical applications [5-7], because it has the capability of closely mimicking the 3D microenvironment as in the human body [8]. More specifically, a kidney-on-a-chip can mimic the structural, mechanical, transport, absorptive, and physiological properties of the human kidney [9]. And to mimic the function of the kidney, mimicking the structure of the unit of the kidney – the nephron, should be first achieved.
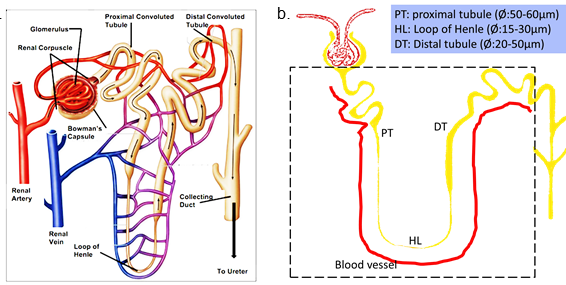
The nephron consists of a renal corpuscle, proximal tubule (PT), loop of Henle (HL), distal tubule (DT), and collecting duct system (Fig.1.a). A simplified experimental design mainly includes the segments of PT, HL and DT as shown in Fig.1.b.
In this project, we focus on mimicking the functional level of kidney lumens including the blood vessels around them, by firstly using 2-photon laser ablation (2PLA) to fabricate these lumens in selective hydrogels (e.g. GelMA):
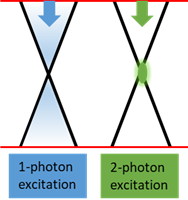
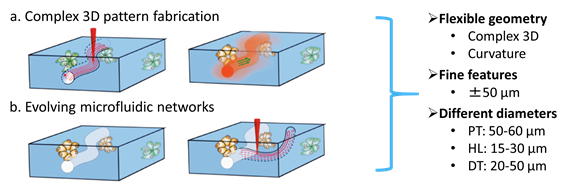
2PLA has been used in creating microfluidic network with micrometer-sized cavities in 3D cell-laden hydrogels [10]. It potentially provides the possibilities of flexible geometry, fine features and easy change in diameter of lumens (Fig.2). Compared to 1-photon excitation, one of the advantages of 2-photon excitation is that the excitation is mostly limited to a small focal volume [11] (Fig.3) which is more suitable for creating fine lumens such as in the nephron.
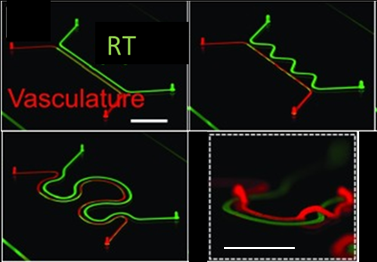
We want to find out which laser exposure dose in terms of scanning speed, voxel size, averaging number and Z-stack numbers (intervals) etc. would be the best option for fabricating lumens which are mostly resemble to those in the nephron, regarding to structure (Fig.4) and functionality.
References
1. Bull. World Health Organ. 96, 414–422C (2018).
2. BMC Public Health. 8, 117 (2008)
3. Kidney Int. Suppl. 3, 368–371 (2013).
4. Nat. Biotechnol. 32, 760–772 (2014).
5. Nat. Rev. Drug Discov. 14, 248–260 (2015).
6. Clin Pharmacol Ther. 101, 31–34 (2017).
7. Biofabrication 8, 015021 (2016).
8. Integr.Biol 5, 1119 (2013).
9. Trends Biotechnol. 34(2):156-170 (2016)
10. Adv. Mater. 28, 7450–7456 (2016).
11. Pflugers Arch. 468(9):1505-16 (2016).
12. Proc Natl Acad Sci U S A. 116(12): 5399-404 (2019)
Contact
-
Ize Uysal Bscmr. Rik Kuipers GEM-Z 3.132Yavuzpad3882CG Oudheusden
-
Mila de Witdrs Johannes Jansen Mscvan der Veldestraat1619KB Alem